 | 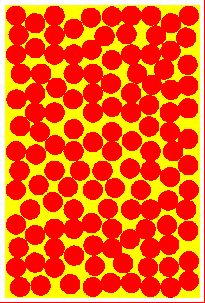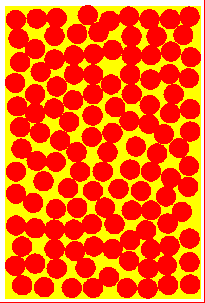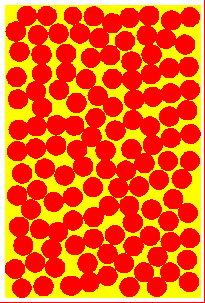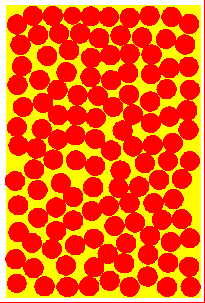 | 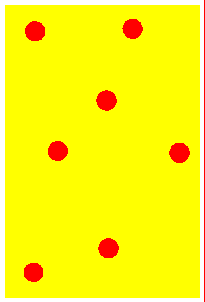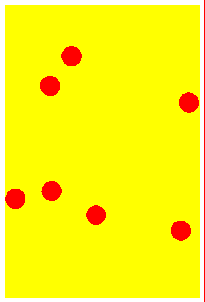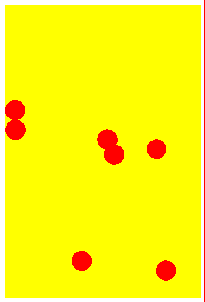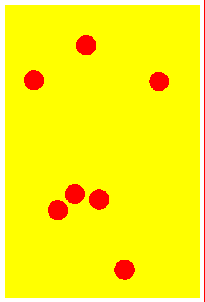 |
Solid At low temperatures, kinetic energy of particles is low. Attractive forces between particles overcome kinetic energy, and the particles 'sit' in their lattice positions, touching each other. Therefore the solid has a definite shape, and is not compressible. Except at absolute zero temperature, the particles merely vibrate about their mean positions because of the small kinetic energy. | Liqid On heating, the kinetic energy of the particles increase. They vibrate more and more vigorously, and at some point will be able to just overcome the attractive forces and move out from the lattice positions. The substance 'melts' into a liquid. Since the particles move, liquids can flow and assume the shape of the container. But while they flow, the particles graze against other particles, giving rise to 'viscosity'. | Gas At very high temperatures, kinetic energy of particles is so much so that the attractive forces between particles is negligible. They move far away from each other and in a completely random manner. There is no shape, and the gas occupies all available space. Since the space between particles is very large, gases are compressible (their volumes decrease when pressed). The energetic collisions with the walls of the container produces pressure. |